Entdeckt in den frühen 90er Jahren durch Dr. Raphael Mechoulam, den Vater der medizinischen Cannabis-Forschung, bildet das Endocannabionid-System die Basis zum Verständnis, wie Cannabis auf unseren Organismus wirkt. Das Endocannabinoid-System ist ein bestimmender Regulator verschiedener Körperfunktionen.
Es gibt kaum einen funktionellen Prozess, der nicht bis zu einem gewissen Grad davon beeinflusst wird.
Trotz der herausragenden Bedeutung des Endocannabinoid-Systems als wesentlicher Regulationsmechanismus in der Biochemie und Physiologie des Körpers sind die Kenntnisse über dieses System nach wie vor recht begrenzt, insbesondere unter deutschen Ärzten.
Gerade hier sehen wir bei der canncura einen hohen Bedarf an Aufklärung sowie Ausbildung.
Unsere ausgebildeten Ärzte werden Sie im Erstgespräch, welches Sie ganz bequem von zu Hause aus digital durchführen können, nicht nur über Cannabis als Medizin, sondern auch über das ECS und dessen Aufgaben aufklären.
Aufbau des ECS
Was sind Cannabinoide?
Phytocannabinoide gehören zur Klasse der Cannabinoide. Diese chemischen Verbindungen kommen in der Cannabis-Pflanze vor. Sie binden an die Cannabinoid-Rezeptoren und nehmen so Einfluss auf die Freisetzung von Botenstoffen im Gehirn. Die zwei bekanntesten Phytocannabinoide sind THC (Tetrahydrocannabinol) und CBD (Cannabidiol).
Endocannabinoide (körpereigene Cannabinoide) gehören ebenfalls zur Klasse der Cannabinoide. Diese werden von unserem Körper hergestellt und imitieren die Wirkungsweise der Phytocannabinoide.
Die zwei primären Endocannabinoide sind AEA (Arachdionylethanolamin), das nach dem Sanskrit-Wort für “Glückseligkeit“ auch Anandamid genannt wird und 2-AG (2-Arachidonylglycerol).
Die Rolle der Endocannabinoide besteht darin, die Homöostase (Gleichgewicht) zu erhalten. Sie sorgen dafür, dass unsere Zellen effektiv, aber nicht übermäßig miteinander kommunizieren.
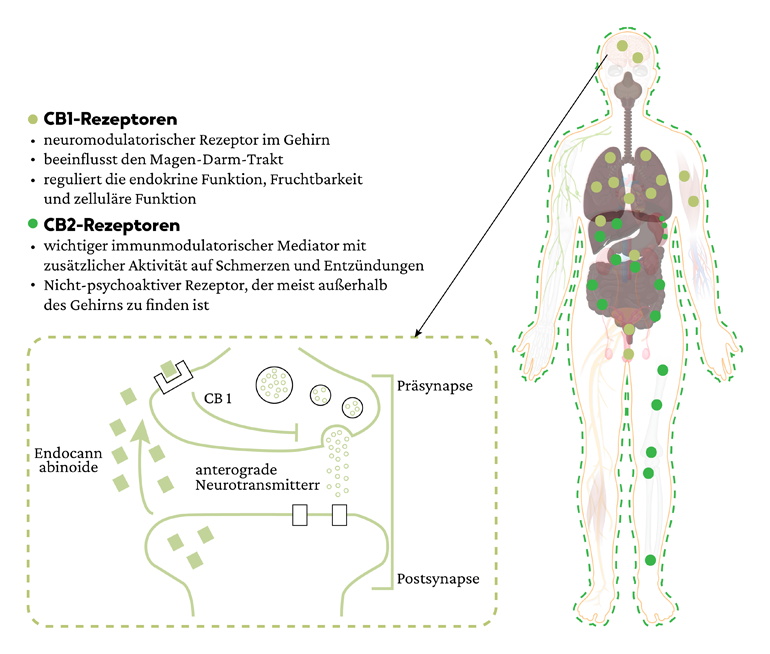
Cannabinoid-Rezeptoren
Im ECS gibt es zwei Hauptrezeptoren – CB1 und CB2.
Einen Rezeptor kann man sich als Schloss vorstellen zu dem ein entsprechender Stoff wie ein Schlüssel (wenn er die richtige Struktur hat) passt. Gelangt der Schlüssel in das Schloss laufen weitere chemische Vorgänge im Zellinneren ab.
CB1-Rezeptoren überwiegen im zentralen Nervensystem, wo sie eine übermäßige Übertragung von Botenstoffen eindämmen. Sobald Nervenzellen eine übermäßige Menge an chemischen Botenstoffen freisetzen, wirken die Endocannabinoide quasi als Bremse, damit eine Balance sichergestellt werden kann.
CB2-Rezeptoren sind im gesamten Körper verteilt, vor allem in den Zellen des Immunsystems und des Magen-Darm-Trakts.
Endocannabinoide aktivieren CB2-Rezeptoren, die sich an den Zellmembranen befinden. Sobald die CB2-Rezeptoren aktiviert sind, lösen sie zahlreiche immunverändernde Wirkungen aus, die von der Art der Zelle und ihrer Umgebung abhängen. Die Aktivierung von CB2-Rezeptoren führt zu einer Reduktion der Freisetzung von entzündlichen Botenstoffen (Zytokinen). Dies hat positive klinische Auswirkungen.
Enzyme
Das ECS steht weitgehend unter enzymatischer Kontrolle. Der Gehalt an Endocannabinoiden wird durch das Gleichgewicht zwischen der Herstellung von Enzymen und deren Abbau bestimmt. Sie sorgen dafür, dass die Endocannabinoide dann verwendet werden, wenn sie gebraucht werden.